Behavioral assays establish zebrafish in drug screening
Zebrafish are increasingly swimming into the view of large-scale drug screening projects. Behavioral screens can be used as a first-line detection tool for new drug effects, and their popularity continues to grow.
Posted by
Published on
Wed 26 Oct. 2011
Topics
| Danio Rerio | EthoVision XT | Video Tracking | Zebrafish |
Zebrafish are increasingly swimming into the view of large-scale drug screening projects. Behavioral screens can be used as a first-line detection tool for new drug effects, and their popularity continues to grow. Translating results to what we might see in humans requires an appropriate (vertebrate) model suited for large-scale studies. This is a tall order, but one zebrafish easily fill. Mice and rats are more established model systems, but size and cost are often prohibitive to large-scale analyses. Compromising in favor of size and cost points to an invertebrate model like Drosophila. However, in zebrafish researchers find the economy required for high throughput studies, in a vertebrate model.
Swimming down the pipeline: behavioral assays establish zebrafish in drug screening
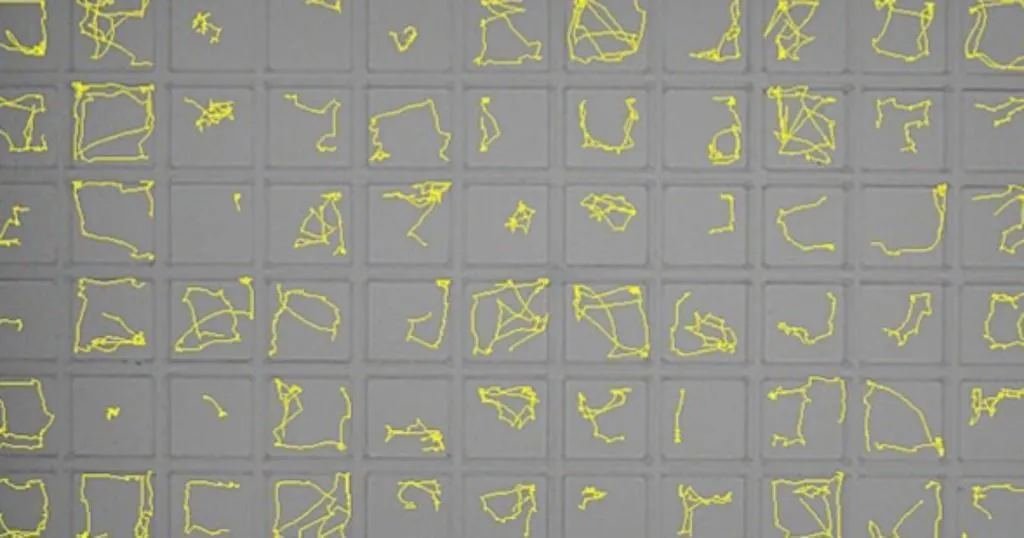
Collwill and Creton (2011) recently reviewed zebrafish behavioral assays and highlight how these can help in discovering new drug targets for anxiety. Successful high throughput screens using zebrafish hinge on using automated imaging, which is a topic touched on in this review. Systems like DanioVision and EthoVision XT have made it possible to track and analyze behavioral traits. This removes the need for time consuming manual scoring, and provides high resolution data gathered in real time.
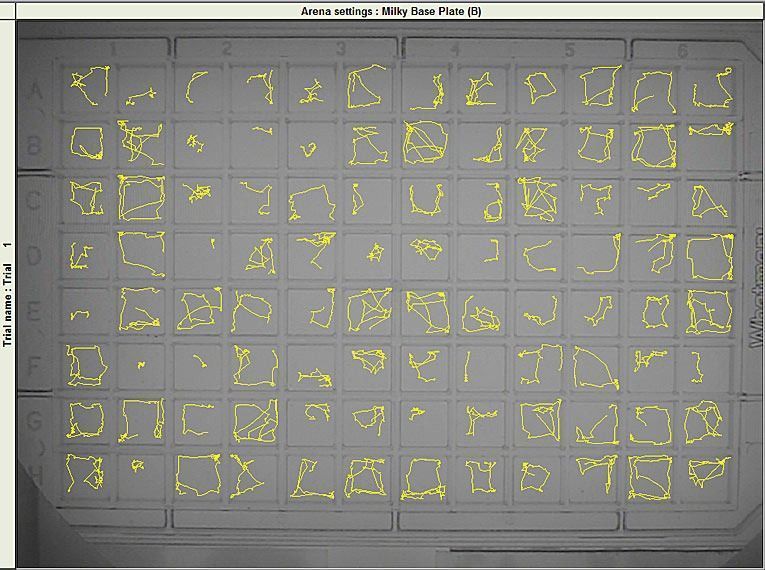
The zebrafish larva’s small size (~4mm) lends itself well to large-scale analysis: One 96 well-plate accommodates an equal number of subjects. Using automated imaging, the average lab can process hundreds of zebrafish a day. For example, EthoVision XT has been employed in isolating seizure-resistant zebrafish larvae in a large-scale mutagenesis screen.
Combining behavioral assays with physiological measures provides an even more powerful avenue for analysis: stress hormones such as cortisol have shown to be elevated in ‘stressed’ zebrafish, essentially providing the basis for validating behavioral data. After observing behavior, it is fairly easy to measure hormone levels, although this is not possible in vivo in zebrafish.
New options for measuring physiological responses during behavioral tasks are heart rate and skin color monitoring. Zebrafish larvae are optically transparent, and a large number of them can be monitored in multiwell plates. Image analysis software can be used to extract specific parameters of the zebrafish heart beat. At this point, imaging heart beat in a freely moving zebrafish is still technically challenging, but once further developed this paradigm can open interesting new avenues for studying the physiology of fear and anxiety.
Large scale screens rely on easily measured observations. For example, thigmotaxis is a behavior that correlates with anxiety. In addition, observing skin color can be very informative: zebrafish larvae adjust their color to their background. Screening for blindness can be as simple as looking at adjustments in color after changing lighting conditions in large-scale studies.
The development of new behavioral assays for zebrafish is in full swing, and the possibilities are vast. The existence of sophisticated behavioral tracking and analysis tools are fueling this growing field of research, and adding to the arsenal of high throughput screens being used in the discovery of new drug targets.
References
Colwill, RM and Creton, R. (2011). Imaging escape and avoidance behavior in zebrafish larvae. Reviews in Neuroscience,22 (1), 63-73.
Related Posts

How zebrafish and optogenetics are great for investigating stress

Zebrafish behaviors shows true effect of chemicals on aquatic animals

